Improving diagnostic and treatment options for Small Cell Lung Cancer
What?
Catherinn BV (Cancer Therapeutics and Innovation) is a virtual research and development company devoted to the improvement of diagnostic and treatment options for Small Cell Lung Cancer (SCLC) patients. Catherinn was started by a group of highly motivated experts and entrepreneurs in the life science and vaccine industry. Important work on SCLC diagnostics and therapy was carried out and patented by the group of prof. Frans Ramaekers, department of molecular cell biology of the Maastricht University Medical Center. We are committed to improving the lives of SCLC cancer patients, both by prevention through early screening and by cure through immunotherapy.
Why?
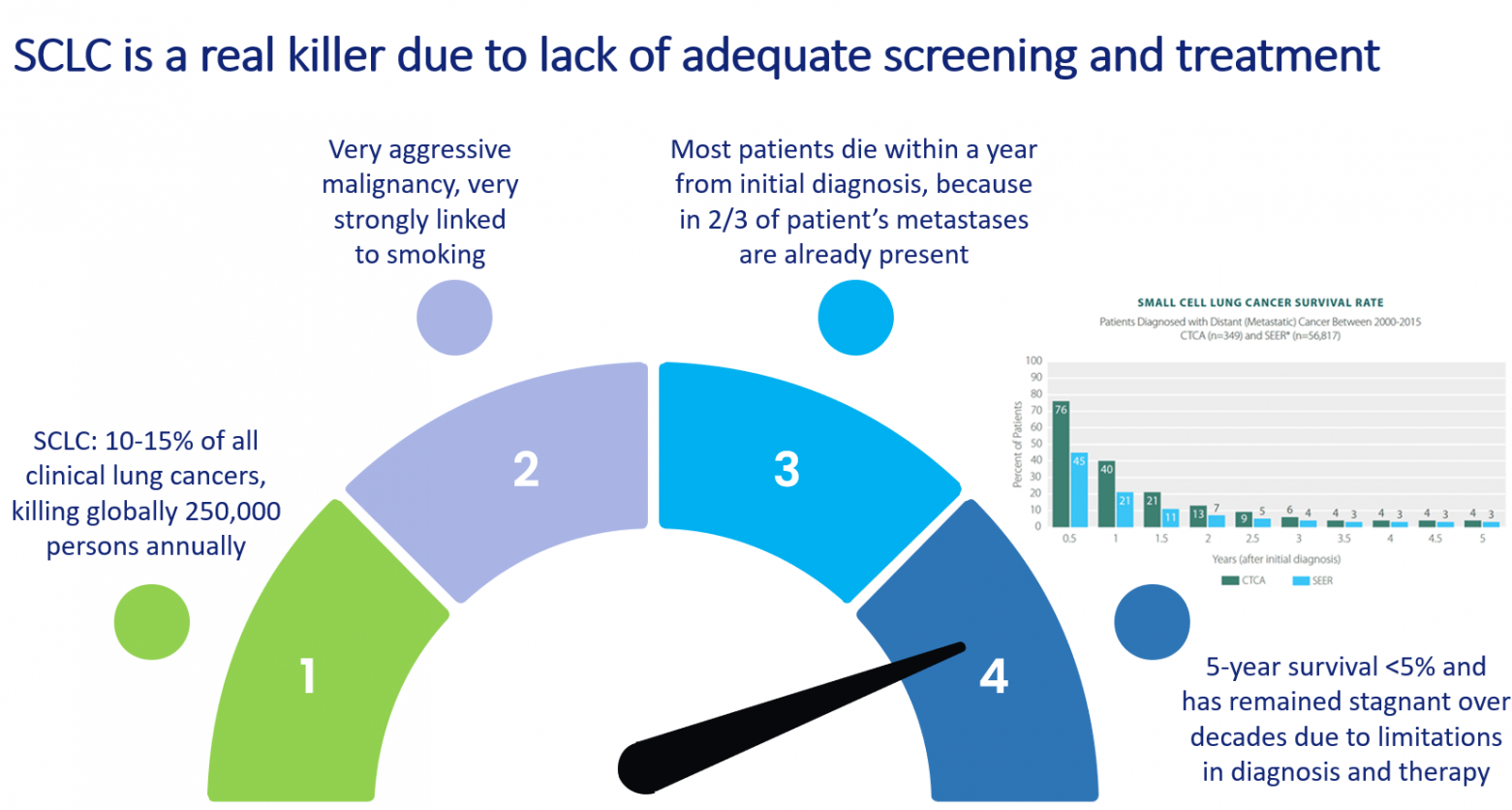
Small Cell Lung Cancer (SCLC) represents 10% to 15% of clinical lung cancer cases. It is an aggressive malignancy strongly associated with smoking, annually killing an estimated 250,000 people worldwide. SCLC is decreasing in industrialized countries due to smoking cessation programs, but it is increasing in low- and middle-income countries.
In about two thirds of the cases SCLC is detected when metastatic lesions are already present. In those cases patients respond well to a first round of chemotherapy, but then invariably resistance to chemotherapy develops and the fast growth rate of resistant tumor cells will lead to death within a year from the initial diagnosis. Over the last decades survival rates for SCLC have not improved.
Therefore, there is a strong medical need for early screening methods and improved therapy.
|
How?
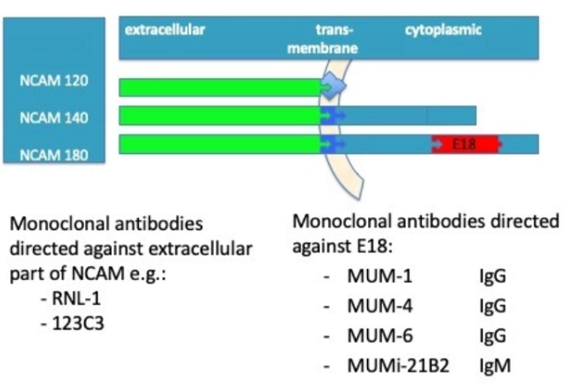
The Neural Cell Adhesion Molecule (NCAM) is a glycoprotein expressed as 120, 140 and/or 180 kDa isoforms, all derived through alternative splicing of a single gene. NCAM 120 contains no intracellular domain, whereas NCAM 140 and 180 have different intracellular domains determined by alternative splicing of exon 18.
We found that NCAM180 is expressed in neuroendrocrine cancer cells (of which SCLC is the major cancer type) and not in other cells, including those of healthy individuals.
This implies that NCAM exon 18 is a biomarker for Small Cell Lung Cancer diagnosis and therapy, where E18 is a Tumor Associated Antigen Specific for SCLC and other Neuroendocrine tumors.
We are looking for collaboration partners for codevelopment to bring our unique and patented NCAM exon 18 approach further. Below you will find diagnostic and treatment options. We would be happy to explore these options with you to make our technology available for your product to improve the lives of the SCLC patients.

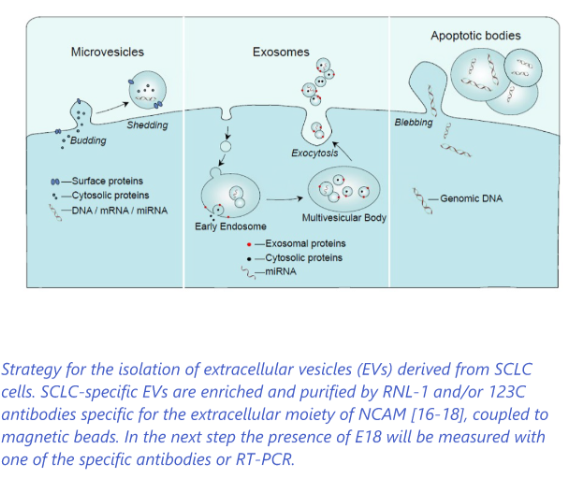
Development of liquid biopsy diagnostic test based on extracellular vesicles (EV) followed by detection of specific SCLC biomarker
- Screening of (ex-) heavy smokers to detect SCLC in an early stage>> improved survival
- Monitoring patients during chemotherapy and after chemotherapy for relapses to guide treatment>> more effective treatment (Companion diagnostics)
|
|

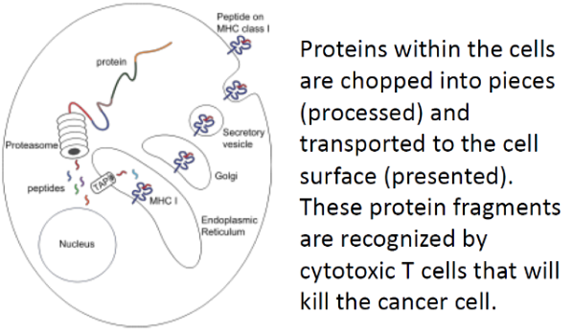
Development of immunotherapy using specific biomarker incorporated onto the proven DC-based immunotherapeutic platform
|
|
Collaboration
Licensing or collaboration opportunities
We are looking for the collaboration or licensing partners who would be interested in developing and commercializing our proven concept further to a diagnostics test for SCLC patients. We are open to idea sharing and future licensing possibilities with respect to the development of SCLC immunotherapy using specific biomarker.
Contact us by mail: thijs@catherinn.com or poonam@catherinn.com and we are happy to provide you with more information.
Latest publications
T-cell responses to small cell lung cancer: the immune system and the tumor are two sides of the same coin
4 August 2023
The 180 splice variant of NCAM--containing exon 18--is specifically expressed in small cell lung cancer cells
6 March 2018
Monoclonal antibodies to the exon 18 encoded moiety of NCAM
20 July 2019
Latest news
Collaboration agreement signed on proof-of-concept SCLC immunotherapy
31 October 2021
First step proof of concept Small Cell Lung Cancer (SCLC) blood test successful
30 September 2021
Business Angel investors finance Catherinn's proof of concept studies on SCLC immunotherapy
15 July 2021
Contact
Please don't hesitate to contact us if you have a question.